This blog post is adapted from a presentation we have given for interested groups who wanted more information about the use of Botox© in Cincinnati. We at Harmon Facial Plastic Surgery recognize the intelligence and sophistication of our clients. They want to understand the treatments they receive at a deeper level. It is important to seek not only a fellowship-trained but also a double board-certified facial plastic surgeon if you have aesthetic concerns about your face and/or neck.

Guide to Botox©
Dr. Jeffrey Harmon
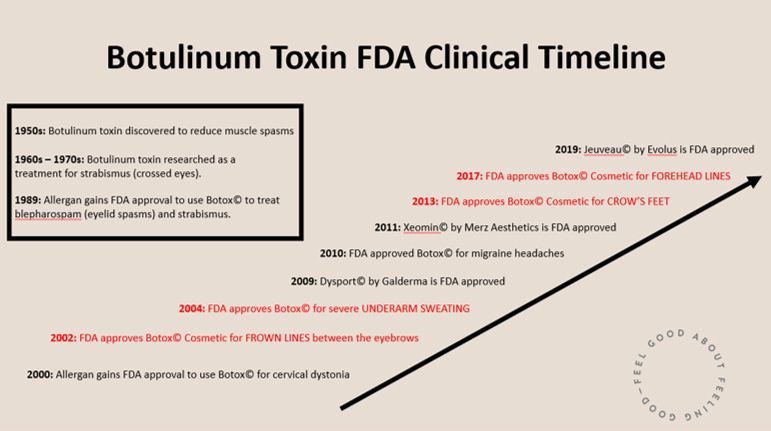
A Brief History of Botox©
Research on the use of botulinum toxin (e.g. Botox©) for therapeutic purposes began in the 1960s for patients with strabismus, or “cross eyes.” It was first FDA-approved for therapeutic use on strabismus in 1989. It was not until 2002 – thirteen years later - that the FDA approved Botox© for cosmetic use. It was first approved for cosmetic use between the eyebrows, also known as the glabella or “eleven lines.” Botox© was then approved for additional areas (underarms, crow’s feet, and forehead) over the next 15 years. Other botulinum toxin products (Xeomin©, Dysport©, Jeuveau©) gained approval for cosmetic use in that period as well.
How Botox© Works
Botox© is delivered at much lower doses for therapeutic and cosmetic purposes than exists naturally. However, its mechanism-of-action is the same. It works at the junction between the muscle and nerve that signals the muscle to contract. Botulinum toxin binds to a terminal at the end of the nerve and prevents the release of molecules that would otherwise signal the muscle to contract. Its effects wear off as the body gradually breaks down and replaces these proteins.
The treatment is effective for dynamic rhytids, or coarse wrinkles that develop when we move our faces. When evaluating the effectiveness of any treatment it is best to find the highest quality studies, which are generally randomized controlled trials (RCTs). Even better are studies that combined the data from multiple RCTs. The Cochrane Library is the gold-standard source of medical research. However, it is not always possible to find research on any subject you desire in this database because of the immense work involved studying each question. Fortunately, The Cochrane Library has published on the effectiveness of botulinum toxin in a paper entitled “Botulinum Toxin Type A for Facial Wrinkles.” They evaluated 65 RCTs that included a total of 14,919 patients. Injection of botulinum toxin in the glabella was compared with placebo. Results ranged from 4 weeks to 2 years after treatment. They concluded that botulinum toxin is likely effective at week 4 after treatment, which is an important finding.
Its effectiveness is reflected in its ubiquity. Botulinum toxin was the most popular non-surgical cosmetic procedure worldwide in 2020 according to statistics compiled by the International Society of Aesthetic Plastic Surgery (ISAPS), with approximately 6.2 million injections performed. The most common age range to receive injections was 35 – 50 years.

Common Questions About Botox©
A common question is whether Botox© administration is painful. A 31-gauge insulin needle is used, the smallest needle size available in clinic. As a result, treatment is generally associated with little-to-no pain.
Another common question is whether Botox© administration is safe. The answer is yes in a significant majority of cases. Its approval by the FDA is an endorsement of its overall safety profile. The best way to evaluate the safety of Botox© treatment is to review both the highest quality data (i.e. RCTs) but also the Adverse Event (AE) reporting to the FDA that occurs whenever a medication is approved for use by the FDA. A 2005 study of FDA-reported AEs associated with botulinum toxin type A used for both therapeutic and cosmetic purposes demonstrated the vast majority of reported AEs at the time were labelled “non-serious,” with 63% of those AEs being a “lack of effect,” 19% involving an injection site reaction (e.g. pain, bruising, swelling, allergic reaction), and 11% involving eyelid ptosis, or a drooping eyelid, which is a temporary possible risk that can be decreased with appropriate technique. A significantly smaller percentage of AEs were labelled as “serious,” the most common of which was swallowing difficulties. It is important to note, however, that reported AEs included injection of botulinum toxin for neck banding, which is in an area very close to muscles that contribute to swallowing. That could explain the above reporting. Moreover, a large majority of AEs reported with botulinum toxin administration were for therapeutic use (e.g. strabismus), not cosmetic use. It is suspected that serious AEs are likely related to both the amount used and the location it is used. Therapeutic uses involve much higher doses than cosmetic uses and are placed in areas with a higher risk of functional problems.
Assessing individuals for possible contraindications and appropriate counseling and informed consent is essential. It is very important to evaluate patients who may not be good candidates for the medication. One example is patients with a history of neuromuscular disorders, including Myasthenia Gravis (MG) and Lambert-Eaton Syndrome (LES). Pregnant and/or nursing mothers should not receive it. Patients actively taking aminoglycoside antibiotics or streptomycin should not receive it because the antibiotics could potentiate the effects of the medication.
Dr. Harmon has a detailed discussion of the potential benefits and risks of injecting Botox© with every client who is interested in treatment. As a fellowship-trained facial plastic surgeon, he has the detailed understanding of facial anatomy necessary to provide these treatments safely and effectively.
Trust Your Face to a Facial Plastic Surgeon
It is important to seek a double board-certified, fellowship-trained specialist in plastic surgery of the face and neck when you have concerns about your face or neck.
Why Choose Dr. Harmon
- The mission of Harmon Facial Plastic Surgery is to help people along their journey towards self-confidence, to feel good about feeling good.
- Dr. Harmon is a double board-certified facial plastic surgeon.
- Dr. Harmon values making patients feel welcomed, listened to, and respected.
- Dr. Harmon graduated with honors from Cornell University with a Bachelor of Science degree in molecular biology.
- Dr. Harmon earned his medical degree from the University of Cincinnati.
- Dr. Harmon underwent five years of extensive training in head at neck surgery at the prestigious residency program at the University of Cincinnati.
- Dr. Harmon then underwent focused fellowship training in cosmetic facial plastic surgery through the American Academy of Facial Plastic and Reconstructive Surgery (AAFPRS) with the world-renowned surgeon, Dr. Andrew Jacono, on Park Avenue in New York City.
Request a Consultation
Request a consultation with Dr. Harmon at Harmon Facial Plastic Surgery in Cincinnati. Visit our clinic. You will learn more about Dr. Harmon’s credentials, style and approach. Build a relationship with our dedicated team. Do not stop at searching “plastic surgery near me.” Get in touch with us today to learn more!
References
- Brin MF, Boodhoo TI, Pogoda JM, James LM, Demos G, Terashima Y, Gu J, Eadie N, Bowen BL. Safety and tolerability of onabotulinumtoxinA in the treatment of facial lines: a meta-analysis of individual patient data from global clinical registration studies in 1678 participants. J Am Acad Dermatol. 2009 Dec;61(6):961-70.e1-11.
- Camargo CP, Xia J, Costa CS, Gemperli R, Tatini MD, Bulsara MK, Riera R. Botulinum toxin type A for facial wrinkles. Cochrane Database Syst Rev. 2021 Jul 5;7(7):CD011301.
- Cohen JL, Scuderi N. Safety and Patient Satisfaction of AbobotulinumtoxinA for Aesthetic Use: A Systematic Review. Aesthet Surg J. 2017 May 1;37(suppl_1):S32-S44.
- Coté TR, Mohan AK, Polder JA, Walton MK, Braun MM. Botulinum toxin type A injections: adverse events reported to the US Food and Drug Administration in therapeutic and cosmetic cases. J Am Acad Dermatol. 2005 Sep;53(3):407-15.
- Guo Y, Lu Y, Liu T, Zhou Y, Yang P, Zhu J, Chen L, Yang Q. Efficacy and Safety of Botulinum Toxin Type A in the Treatment of Glabellar Lines: A Meta-Analysis of Randomized, Placebo-Controlled, Double-Blind Trials. Plast Reconstr Surg. 2015 Sep;136(3):310e-318e.
- Jia Z, Lu H, Yang X, Jin X, Wu R, Zhao J, Chen L, Qi Z. Adverse Events of Botulinum Toxin Type A in Facial Rejuvenation: A Systematic Review and Meta-Analysis. Aesthetic Plast Surg. 2016 Oct;40(5):769-77.
- Sundaram H, Signorini M, Liew S, Trindade de Almeida AR, Wu Y, Vieira Braz A, Fagien S, Goodman GJ, Monheit G, Raspaldo H; Global Aesthetics Consensus Group. Global Aesthetics Consensus: Botulinum Toxin Type A--Evidence-Based Review, Emerging Concepts, and Consensus Recommendations for Aesthetic Use, Including Updates on Complications. Plast Reconstr Surg. 2016 Mar;137(3):518e-529e.
This blog post is for educational purposes only and does not constitute direct medical advice. It is essential that you have a consultation with a qualified medical provider prior to considering any treatment. This will allow you the opportunity to discuss any potential benefits, risks, and alternatives to the treatment.
